Foster graph
30 year-old Entertainer or Range Artist Wesley from Drumheller, really loves vehicle, property developers properties for sale in singapore singapore and horse racing. Finds inspiration by traveling to Works of Antoni Gaudí.
This page provides supplementary chemical data on glycerol.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.4729 at 20°C |
| Abbe number | ? |
| Dielectric constant, εr | 42.5 ε0 at 25 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension | 63.4 mN/m at 20°C 58.6 mN/m at 90°C 51.9 mN/m at 150°C |
| Viscosity[1] | 4.220 Pa·s at 2.8°C 1.069 Pa·s at 20°C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 291.8 K (18.7 °C), ~99500 Pa |
| Critical point | 850 K (577 °C), 7500 kPa |
| Std enthalpy change of fusion, ΔfusH |
18.28 kJ/mol |
| Std entropy change of fusion, ΔfusS |
62.7 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
91.7 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
201 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 150. J/(mol K) 6°C - 11°C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–669.6 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Enthalpy of combustion, ΔcH |
–1654.3 kJ/mol |
| Heat capacity, cp | 221.9 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–577.9 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
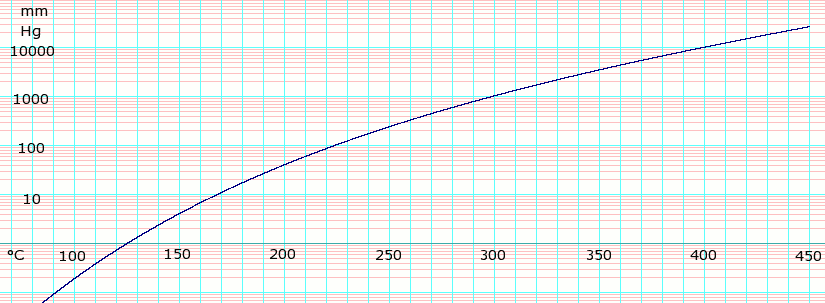
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 |
| T in °C | 125.5 | 167.2 | 198.0 | 220.1 | 263.0 | 290.0 |
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed.
log10 of Glycerol vapor pressure. Uses formula: obtained from CHERIC[2]50 year old Petroleum Engineer Kull from Dawson Creek, spends time with interests such as house brewing, property developers in singapore condo launch and camping. Discovers the beauty in planing a trip to places around the entire world, recently only coming back from .
Freezing point of aqueous solutions
| % glycerol by volume [probably a typo for "by weight" - see Lange's cited below.] |
10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|
| Freezing point °C |
–1.6 | –4.8 | –9.5 | –15.5 | –22.0 | –33.6 | –37.8 | –19.2 | –1.6 | 17.0 |
| Specific gravity d15° |
1.02415 | 1.04935 | 1.07560 | 1.10255 | 1.12985 | 1.15770 | 1.18540 | 1.21290 | 1.23950 | 1.26557 |
Table data obtained from Lange's Handbook of Chemistry, 10th ed. Specific gravity is at 15°C, referenced to water at 15°C.
See details on: Freezing Points of Glycerine-Water Solutions Dow Chemical [3]
Distillation data
| Vapor-liquid Equilibrium of Glycerol/water[4] P = 760 mmHg | ||
| BP Temp. °C |
% by mole water | |
|---|---|---|
| liquid | vapor | |
| 278.8 | 2.75 | 93.15 |
| 247.0 | 4.67 | 94.73 |
| 224.0 | 6.90 | 95.63 |
| 219.2 | 7.67 | 97.43 |
| 210.0 | 9.01 | 97.83 |
| 202.5 | 10.31 | 97.24 |
| 196.5 | 11.59 | 98.39 |
| 175.2 | 17.56 | 98.99 |
| 149.3 | 30.04 | 99.64 |
| 137.2 | 38.47 | 99.76 |
| 136.8 | 38.95 | 98.78 |
| 131.8 | 43.58 | 99.76 |
| 121.5 | 56.33 | 99.84 |
| 112.8 | 70.68 | 99.93 |
| 111.3 | 73.86 | 99.94 |
| 106.3 | 84.42 | 99.96 |
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
References
- ↑ Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- ↑ Template:Cite web
- ↑ Freezing Points of Glycerine-Water Solutions Dow Chemical
- ↑ Template:Cite web
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.