Figure-eight knot (mathematics): Difference between revisions
en>Momotaro +explanation |
en>EuroCarGT m Reverted edits by 146.135.35.216 (talk) to last revision by Atethnekos (HG) |
||
| Line 1: | Line 1: | ||
{{more footnotes|date=December 2008}} | |||
[[File:First Ionization Energy.svg|thumb|500px|Periodic trend for ionization energy. Each period begins at a minimum for the alkali metals, and ends at a maximum for the noble gases.]] | |||
The '''ionization energy''' ('''IE''') of an [[atom]] or [[molecule]] describes the minimum amount of energy required to remove an electron (to infinity) from the atom or molecule in the [[gas]]eous state<ref>http://www.princeton.edu/~achaney/tmve/wiki100k/docs/Ionization_potential.html</ref>. | |||
::X + energy → X<sup>+</sup> + e<sup>-</sup> | |||
The term '''ionization potential''' has been used in the past but is not recommended.<ref name="Muller1994">{{cite journal|last1=Muller|first1=P.|title=Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994)|journal=Pure and Applied Chemistry|volume=66|issue=5|year=1994|pages=1077–1184|issn=0033-4545|doi=10.1351/pac199466051077}}</ref> | |||
The units for ionization energy vary from discipline to discipline. In [[physics]], the ionization energy is typically specified in [[electron volt]]s (eV) and refers to the energy required to remove a single electron from a single atom or molecule. In [[chemistry]], the ionization energy is typically specified as a [[Mole (unit)|molar]] quantity (molar ionization energy or [[enthalpy]]) and is reported in units of kJ/mol or kcal/mol (the amount of energy it takes for all the atoms in a mole to lose one electron each<ref>http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Table_of_the_Elements/Ionization_Energy</ref>). | |||
The ''n''<sup>th</sup> ionization energy refers to the amount of energy required to remove an electron from the species with a charge of (''n''-1). For example, the first three ionization energies are defined as follows: | |||
::1<sup>st</sup> ionization energy | |||
:::X → X<sup>+</sup> + e<sup>-</sup> | |||
::2<sup>nd</sup> ionization energy | |||
:::X<sup>+</sup> → X<sup>2+</sup> + e<sup>-</sup> | |||
::3<sup>rd</sup> ionization energy | |||
:::X<sup>2+</sup> → X<sup>3+</sup> + e<sup>-</sup> | |||
== Values and trends == | |||
[[File:Electron binding energy vs Z.jpg|thumb|500px]] | |||
{{main|Molar ionization energies of the elements|Ionization energies of the elements (data page)}} | |||
Generally the (''n''+1)th ionization energy is larger than the ''n''th ionization energy. Always, the next ionization energy involves removing an electron from an orbital closer to the nucleus. Electrons in the closer orbitals experience greater forces of electrostatic attraction; thus, their removal requires increasingly more energy. Ionization energy becomes greater up and to the right of the periodic table. | |||
Some values for elements of the third period are given in the following table: | |||
{| cell padding="4" class="wikitable sortable" | |||
|+''Successive molar ionization energies in'' [[joule|kJ]]/[[mole (unit)|mol]] <br> (96.485 kJ/mol = 1 [[electron volt|eV]]/particle) | |||
|- | |||
! Element | |||
! First | |||
! Second | |||
! Third | |||
! Fourth | |||
! Fifth | |||
! Sixth | |||
! Seventh | |||
|- align="right" | |||
! align="center" | [[Sodium|Na]] | |||
| 496 | |||
| 4,560 || || || || || | |||
|- align="right" | |||
! align="center" | [[Magnesium|Mg]] | |||
| 738 | |||
| 1,450 | |||
| 7,730 || || || || | |||
|- align="right" | |||
! align="center" | [[Aluminium|Al]] | |||
| 577 | |||
| 1,816 | |||
| 2,881 | |||
| 11,600 || || || | |||
|- align="right" | |||
! align="center" | [[Silicon|Si]] | |||
| 786 | |||
| 1,577 | |||
| 3,228 | |||
| 4,354 | |||
| 16,100 || || | |||
|- align="right" | |||
! align="center" | [[Phosphorus|P]] | |||
| 1,060 | |||
| 1,890 | |||
| 2,905 | |||
| 4,950 | |||
| 6,270 | |||
| 21,200 || | |||
|- align="right" | |||
! align="center" | [[Sulfur|S]] | |||
| 999.6 | |||
| 2,260 | |||
| 3,375 | |||
| 4,565 | |||
| 6,950 | |||
| 8,490 | |||
| 27,107 | |||
|- align="right" | |||
! align="center" | [[Chlorine|Cl]] | |||
| 1,256 | |||
| 2,295 | |||
| 3,850 | |||
| 5,160 | |||
| 6,560 | |||
| 9,360 | |||
| 11,000 | |||
|- align="right" | |||
! align="center" | [[Argon|Ar]] | |||
| 1,520 | |||
| 2,665 | |||
| 3,945 | |||
| 5,770 | |||
| 7,230 | |||
| 8,780 | |||
| 12,000 | |||
|} | |||
Large jumps in the successive molar ionization energies occur when passing [[noble gas]] configurations. For example, as can be seen in the table above, the first two molar ionization energies of magnesium (stripping the two 3s electrons from a magnesium atom) are much smaller than the third, which requires stripping off a 2p electron from the very stable [[neon]] configuration of Mg<sup>2+</sup>. | |||
Ionization energy is also a periodic trend within the [[periodic table]] organization. Moving left to right within a [[Period (periodic table)|period]] or upward within a [[Group (periodic table)|group]], the first ionization energy generally increases with a few discrepancies (aluminum and sulphur). As the nuclear charge of the nucleus increases across the period, the [[atomic radius]] decreases and the electron cloud becomes closer towards the nucleus. | |||
Ionization energy increases from left to right in a period and decreases from top to bottom in a group. | |||
== Electrostatic explanation == | |||
Atomic ionization energy can be predicted by an analysis using [[electrostatic potential]] and the [[Bohr model]] of the atom, as follows (note that the derivation uses [[Gaussian units]]). | |||
Consider an electron of charge ''-[[elementary charge|e]]'' and an atomic nucleus with charge ''+Ze'', where ''Z'' is the number of protons in the nucleus. According to the [[Bohr model]], if the electron were to approach and bind with the atom, it would come to rest at a certain radius ''a''. The electrostatic potential ''V'' at distance ''a'' from the ionic nucleus, referenced to a point infinitely far away, is: | |||
<math>V = \frac{Ze}{a} \,\!</math> | |||
Since the electron is negatively charged, it is drawn inwards by this positive electrostatic potential. The energy required for the electron to "climb out" and leave the atom is: | |||
<math>E = eV = \frac{Ze^2}{a} \,\!</math> | |||
This analysis is incomplete, as it leaves the distance ''a'' as an unknown variable. It can be made more rigorous by assigning to each electron of every [[chemical element]] a characteristic distance, chosen so that this relation agrees with experimental data. | |||
It is possible to expand this model considerably by taking a semi-classical approach, in which momentum is quantized. This approach works very well for the hydrogen atom, which only has one electron. The magnitude of the angular momentum for a circular orbit is: | |||
<math> L = |\mathbf r \times \mathbf p| = rmv = n\hbar</math> | |||
The total energy of the atom is the sum of the kinetic and potential energies, that is: | |||
<math> E = T + U = \frac{p^2}{2m_e} - \frac{Ze^2}{r} = \frac{m_e v^2}{2} - \frac{Ze^2}{r}</math> | |||
Velocity can be eliminated from the kinetic energy term by setting the Coulomb attraction equal to the centripetal force, giving: | |||
<math> T = \frac{Ze^2}{2r}</math> | |||
Solving the angular momentum for ''v'' and substituting this into the expression for kinetic energy, we have: | |||
<math>\frac{n^2 \hbar^2}{rm_e} = Ze^2</math> | |||
This establishes the dependence of the radius on ''n''. That is: | |||
<math>r(n) = \frac{n^2 \hbar^2}{Zm_e e^2}</math> | |||
Now the energy can be found in terms of ''Z'', ''e'', and ''r''. Using the new value for the kinetic energy in the total energy equation above, it is found that: | |||
<math>E = - \frac{Ze^2}{2r}</math> | |||
At its smallest value, ''n'' is equal to 1 and ''r'' is the [[Bohr radius]] ''a<sub>0</sub>'' which equals to <math> \frac{\hbar^2}{m e^2}</math>. Now, the equation for the energy can be established in terms of the Bohr radius. Doing so gives the result: | |||
<math> E = - \frac{1}{n^2} \frac{Z^2e^2}{2a_0} = - \frac{Z^213.6eV}{n^2}</math> | |||
==Quantum-mechanical explanation== | |||
According to the more complete theory of [[quantum mechanics]], the location of an electron is best described as a probability distribution. The energy can be calculated by integrating over this cloud. The cloud's underlying mathematical representation is the [[wavefunction]] which is built from [[Slater determinant]]s consisting of molecular spin orbitals. These are related by [[Pauli's exclusion principle]] to the antisymmetrized products of the [[atomic orbital|atomic]] or [[molecular orbitals]]. | |||
In general, calculating the ''n''th ionization energy requires calculating the energies of <math>Z-n+1</math> and <math>Z-n</math> electron systems. Calculating these energies exactly is not possible except for the simplest systems (i.e. hydrogen), primarily because of difficulties in integrating the [[electron correlation]] terms. Therefore, approximation methods are routinely employed, with different methods varying in complexity (computational time) and in accuracy compared to empirical data. This has become a well-studied problem and is routinely done in [[computational chemistry]]. At the lowest level of approximation, the ionization energy is provided by [[Koopmans' theorem]]. | |||
==Vertical and adiabatic ionization energy in molecules== | |||
[[File:Franck-Condon-diagram.png|280px|thumb|'''Figure 1.''' Franck–Condon principle energy diagram. For ionization of a diatomic molecule the only nuclear coordinate is the bond length. The lower curve is the [[Potential energy surface|potential energy curve]] of the neutral molecule, and the upper curve is for the positive ion with a longer bond length. The blue arrow is vertical ionization, here from the ground state of the molecule to the v=2 level of the ion.]] | |||
Ionization of molecules often leads to changes in [[molecular geometry]], and two types of (first) ionization energy are defined – ''adiabatic'' and ''vertical''.<ref>[http://cccbdb.nist.gov/adiabatic.asp The difference between a vertical ionization energy and adiabatic ionization energy.]</ref> | |||
Adiabatic ionization energy: The [[adiabatic theorem|adiabatic]] ionization energy of a molecule is the ''minimum'' amount of energy required to remove an electron from a neutral molecule, i.e. the difference between the energy of the [[molecular vibration|vibrational]] [[ground state]] of the neutral species and that of the positive ion. The specific equilibrium geometry of each species does not affect this value. | |||
Vertical ionization energy: Due to the possible changes in molecular geometry that may result from ionization, additional transitions may exist between the vibrational ground state of the neutral species and [[molecular vibration|vibrational]] [[excited state]]s of the positive ion. In other words, ionization is accompanied by [[vibrational spectroscopy|vibrational excitation]]. The intensity of such transitions are explained by the [[Franck–Condon principle]], which predicts that the most probable and intense transition corresponds to the vibrational excited state of the positive ion that has the same geometry as the neutral molecule. This transition is referred to as the "vertical" ionization energy since it is represented by a completely vertical line on a potential energy diagram (see Figure). | |||
For a diatomic molecule, the geometry is defined by the length of a single [[bond length|bond]]. The removal of an electron from a bonding [[molecular orbital]] weakens the bond and increases the bond length. In Figure 1, the lower [[Potential energy surface|potential energy curve]] is for the neutral molecule and the upper surface is for the positive ion. Both curves plot the potential energy as a function of bond length. The horizontal lines correspond to [[Molecular vibration|vibrational levels]] with their associated [[Quantum harmonic oscillator|vibrational wave functions]]. Since the ion has a weaker bond, it will have a longer bond length. This effect is represented by shifting the minimum of the potential energy curve to the right of the neutral species. The adiabatic ionization is the diagonal transition to the vibrational ground state of the ion. Vertical ionization involves vibrational excitation of the ionic state and therefore requires greater energy. | |||
In many circumstances, the adiabatic ionization energy is often a more desirable physical quantity since it describes the difference in energy between the two potential energy surfaces. However, due to experimental limitations, the adiabatic ionization energy is often difficult to determine, whereas the vertical detachment energy is easily identifiable and measurable. | |||
==Analogs of Ionization Energy to Other Systems== | |||
While the term ionization energy is largely used only for gas-phase atomic or molecular species, there are a number of analogous quantities that consider the amount of energy required to remove an electron from other physical systems. | |||
Electron binding energy: A generic term for the ionization energy that can be used for species with any charge state. For example, the electron binding energy for the chloride ion is the minimum amount of energy required to remove an electron from the chlorine atom when it has a charge of -1. In this particular example, the electron binding energy has the same magnitude as the electron affinity for the neutral chlorine atom. In another example, the electron binding energy refers the minimum amount of energy required to remove an electron from the dicarboxylate dianion <sup>-</sup>O<sub>2</sub>C(CH<sub>2</sub>)<sub>8</sub>CO<sub>2</sub><sup>-</sup>. | |||
[[Work function]]: The minimum amount of energy required to remove an electron from a solid surface. | |||
==See also== | |||
* [[Electron affinity]] — a closely related concept describing the energy released by ''adding'' an electron to a neutral atom or molecule. | |||
* [[Work function]] is the energy required to strip an electron from a [[solid]] to just outside its surface. | |||
* [[Electronegativity]] is a number that shares some similarities with ionization energy. | |||
* [[Koopmans' theorem]], regarding the predicted ionization energies in [[Hartree–Fock]] theory. | |||
* [[Di-tungsten tetra(hpp)]] has the lowest recorded ionization energy for a stable [[chemical compound]]. | |||
==References== | |||
{{Reflist}} | |||
{{DEFAULTSORT:Ionization Energy}} | |||
[[Category:Ions]] | |||
[[Category:Molecular physics]] | |||
[[Category:Atomic physics]] | |||
[[Category:Chemical properties]] | |||
[[Category:Quantum chemistry]] | |||
Revision as of 20:23, 14 October 2013

The ionization energy (IE) of an atom or molecule describes the minimum amount of energy required to remove an electron (to infinity) from the atom or molecule in the gaseous state[1].
- X + energy → X+ + e-
The term ionization potential has been used in the past but is not recommended.[2]
The units for ionization energy vary from discipline to discipline. In physics, the ionization energy is typically specified in electron volts (eV) and refers to the energy required to remove a single electron from a single atom or molecule. In chemistry, the ionization energy is typically specified as a molar quantity (molar ionization energy or enthalpy) and is reported in units of kJ/mol or kcal/mol (the amount of energy it takes for all the atoms in a mole to lose one electron each[3]).
The nth ionization energy refers to the amount of energy required to remove an electron from the species with a charge of (n-1). For example, the first three ionization energies are defined as follows:
- 1st ionization energy
- X → X+ + e-
- 1st ionization energy
- 2nd ionization energy
- X+ → X2+ + e-
- 2nd ionization energy
- 3rd ionization energy
- X2+ → X3+ + e-
- 3rd ionization energy
Values and trends
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church.
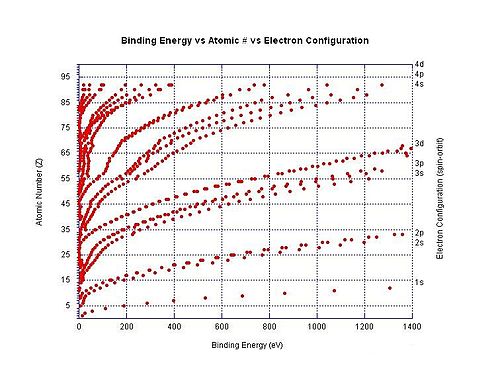
Generally the (n+1)th ionization energy is larger than the nth ionization energy. Always, the next ionization energy involves removing an electron from an orbital closer to the nucleus. Electrons in the closer orbitals experience greater forces of electrostatic attraction; thus, their removal requires increasingly more energy. Ionization energy becomes greater up and to the right of the periodic table.
Some values for elements of the third period are given in the following table:
| Element | First | Second | Third | Fourth | Fifth | Sixth | Seventh |
|---|---|---|---|---|---|---|---|
| Na | 496 | 4,560 | |||||
| Mg | 738 | 1,450 | 7,730 | ||||
| Al | 577 | 1,816 | 2,881 | 11,600 | |||
| Si | 786 | 1,577 | 3,228 | 4,354 | 16,100 | ||
| P | 1,060 | 1,890 | 2,905 | 4,950 | 6,270 | 21,200 | |
| S | 999.6 | 2,260 | 3,375 | 4,565 | 6,950 | 8,490 | 27,107 |
| Cl | 1,256 | 2,295 | 3,850 | 5,160 | 6,560 | 9,360 | 11,000 |
| Ar | 1,520 | 2,665 | 3,945 | 5,770 | 7,230 | 8,780 | 12,000 |
Large jumps in the successive molar ionization energies occur when passing noble gas configurations. For example, as can be seen in the table above, the first two molar ionization energies of magnesium (stripping the two 3s electrons from a magnesium atom) are much smaller than the third, which requires stripping off a 2p electron from the very stable neon configuration of Mg2+.
Ionization energy is also a periodic trend within the periodic table organization. Moving left to right within a period or upward within a group, the first ionization energy generally increases with a few discrepancies (aluminum and sulphur). As the nuclear charge of the nucleus increases across the period, the atomic radius decreases and the electron cloud becomes closer towards the nucleus.
Ionization energy increases from left to right in a period and decreases from top to bottom in a group.
Electrostatic explanation
Atomic ionization energy can be predicted by an analysis using electrostatic potential and the Bohr model of the atom, as follows (note that the derivation uses Gaussian units).
Consider an electron of charge -e and an atomic nucleus with charge +Ze, where Z is the number of protons in the nucleus. According to the Bohr model, if the electron were to approach and bind with the atom, it would come to rest at a certain radius a. The electrostatic potential V at distance a from the ionic nucleus, referenced to a point infinitely far away, is:
Since the electron is negatively charged, it is drawn inwards by this positive electrostatic potential. The energy required for the electron to "climb out" and leave the atom is:
This analysis is incomplete, as it leaves the distance a as an unknown variable. It can be made more rigorous by assigning to each electron of every chemical element a characteristic distance, chosen so that this relation agrees with experimental data.
It is possible to expand this model considerably by taking a semi-classical approach, in which momentum is quantized. This approach works very well for the hydrogen atom, which only has one electron. The magnitude of the angular momentum for a circular orbit is:
The total energy of the atom is the sum of the kinetic and potential energies, that is:
Velocity can be eliminated from the kinetic energy term by setting the Coulomb attraction equal to the centripetal force, giving:
Solving the angular momentum for v and substituting this into the expression for kinetic energy, we have:
This establishes the dependence of the radius on n. That is:
Now the energy can be found in terms of Z, e, and r. Using the new value for the kinetic energy in the total energy equation above, it is found that:
At its smallest value, n is equal to 1 and r is the Bohr radius a0 which equals to . Now, the equation for the energy can be established in terms of the Bohr radius. Doing so gives the result:
Quantum-mechanical explanation
According to the more complete theory of quantum mechanics, the location of an electron is best described as a probability distribution. The energy can be calculated by integrating over this cloud. The cloud's underlying mathematical representation is the wavefunction which is built from Slater determinants consisting of molecular spin orbitals. These are related by Pauli's exclusion principle to the antisymmetrized products of the atomic or molecular orbitals.
In general, calculating the nth ionization energy requires calculating the energies of and electron systems. Calculating these energies exactly is not possible except for the simplest systems (i.e. hydrogen), primarily because of difficulties in integrating the electron correlation terms. Therefore, approximation methods are routinely employed, with different methods varying in complexity (computational time) and in accuracy compared to empirical data. This has become a well-studied problem and is routinely done in computational chemistry. At the lowest level of approximation, the ionization energy is provided by Koopmans' theorem.
Vertical and adiabatic ionization energy in molecules

Ionization of molecules often leads to changes in molecular geometry, and two types of (first) ionization energy are defined – adiabatic and vertical.[4]
Adiabatic ionization energy: The adiabatic ionization energy of a molecule is the minimum amount of energy required to remove an electron from a neutral molecule, i.e. the difference between the energy of the vibrational ground state of the neutral species and that of the positive ion. The specific equilibrium geometry of each species does not affect this value.
Vertical ionization energy: Due to the possible changes in molecular geometry that may result from ionization, additional transitions may exist between the vibrational ground state of the neutral species and vibrational excited states of the positive ion. In other words, ionization is accompanied by vibrational excitation. The intensity of such transitions are explained by the Franck–Condon principle, which predicts that the most probable and intense transition corresponds to the vibrational excited state of the positive ion that has the same geometry as the neutral molecule. This transition is referred to as the "vertical" ionization energy since it is represented by a completely vertical line on a potential energy diagram (see Figure).
For a diatomic molecule, the geometry is defined by the length of a single bond. The removal of an electron from a bonding molecular orbital weakens the bond and increases the bond length. In Figure 1, the lower potential energy curve is for the neutral molecule and the upper surface is for the positive ion. Both curves plot the potential energy as a function of bond length. The horizontal lines correspond to vibrational levels with their associated vibrational wave functions. Since the ion has a weaker bond, it will have a longer bond length. This effect is represented by shifting the minimum of the potential energy curve to the right of the neutral species. The adiabatic ionization is the diagonal transition to the vibrational ground state of the ion. Vertical ionization involves vibrational excitation of the ionic state and therefore requires greater energy.
In many circumstances, the adiabatic ionization energy is often a more desirable physical quantity since it describes the difference in energy between the two potential energy surfaces. However, due to experimental limitations, the adiabatic ionization energy is often difficult to determine, whereas the vertical detachment energy is easily identifiable and measurable.
Analogs of Ionization Energy to Other Systems
While the term ionization energy is largely used only for gas-phase atomic or molecular species, there are a number of analogous quantities that consider the amount of energy required to remove an electron from other physical systems.
Electron binding energy: A generic term for the ionization energy that can be used for species with any charge state. For example, the electron binding energy for the chloride ion is the minimum amount of energy required to remove an electron from the chlorine atom when it has a charge of -1. In this particular example, the electron binding energy has the same magnitude as the electron affinity for the neutral chlorine atom. In another example, the electron binding energy refers the minimum amount of energy required to remove an electron from the dicarboxylate dianion -O2C(CH2)8CO2-.
Work function: The minimum amount of energy required to remove an electron from a solid surface.
See also
- Electron affinity — a closely related concept describing the energy released by adding an electron to a neutral atom or molecule.
- Work function is the energy required to strip an electron from a solid to just outside its surface.
- Electronegativity is a number that shares some similarities with ionization energy.
- Koopmans' theorem, regarding the predicted ionization energies in Hartree–Fock theory.
- Di-tungsten tetra(hpp) has the lowest recorded ionization energy for a stable chemical compound.
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
- ↑ http://www.princeton.edu/~achaney/tmve/wiki100k/docs/Ionization_potential.html
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Table_of_the_Elements/Ionization_Energy
- ↑ The difference between a vertical ionization energy and adiabatic ionization energy.











