Carathéodory metric: Difference between revisions
en>Mathsci |
en>Yobot m WP:CHECKWIKI error fixes / special characters in sortkey fixed using AWB (9427) |
||
| Line 1: | Line 1: | ||
{{Use dmy dates|date=June 2013}} | |||
<!-- To obtain a blank version of this page, type subst:chembox supplement inside of double curly braces, {{}}, and save the page --> | |||
This page provides supplementary chemical data on [[1,2-dichloroethane]]. <!-- replace with proper wikilink --> | |||
== Structure and properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Structure and properties | |||
|- | |||
| [[Index of refraction]],<ref>{{Cite web|url=http://www.chemicalland21.com/industrialchem/organic/ETHYLENE%20DICHLORIDE.htm|title=Ethylene Dichloride|publisher=ChemicalLand21|accessdate=4 June 2007| archiveurl= http://web.archive.org/web/20070609153344/http://www.chemicalland21.com/industrialchem/organic/ETHYLENE%20DICHLORIDE.htm| archivedate= 9 June 2007 <!--Added by DASHBot-->}}</ref> ''n''<sub>D</sub> | |||
| 1.4448 <!-- Please omit if not applicable --> | |||
|- | |||
| [[Abbe number]] | |||
|? <!-- Please omit if not applicable --> | |||
|- | |||
| [[Dielectric constant]],<ref>{{Cite web|url=http://www.asiinstr.com/technical/Dielectric%20Constants.htm|title=Dielectric Constants Chart|publisher=ASI Instruments|accessdate=4 June 2007| archiveurl= http://web.archive.org/web/20070529185028/http://www.asiinstr.com/technical/Dielectric%20Constants.htm| archivedate= 29 May 2007 <!--Added by DASHBot-->}}</ref> ε<sub>r</sub> | |||
| 10.5 ε<sub>0</sub> at 20 °C <!-- Please omit if not applicable --> | |||
|- | |||
| [[Bond strength]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond length]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond angle]] | |||
| ? <!-- Specify which angle, e.g. Cl-P-O. Please omit if not applicable --> | |||
|- | |||
| [[Magnetic susceptibility]] | |||
| ? <!-- Please omit if not applicable --> | |||
|- | |||
| [[Surface tension]]<ref>''Lange's Handbook of Chemistry'', 10th ed. pp 1161-1163</ref> | |||
| 40.05 mN/m at 10°C<br>38.75 mN/m at 20°C<br>28.4 mN/m at 100°C | |||
|- | |||
| [[Viscosity]]<ref>''Lange's Handbook of Chemistry'', 10th ed. pp 1669-1674</ref> | |||
| 1.1322 mPa·s at 0°C<br>0.8385 mPa·s at 20°C<br>0.6523 mPa·s at 40°C<br>0.4357 mPa·s at 80°C | |||
|- | |||
|} | |||
== Thermodynamic properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Phase behavior | |||
|- | |||
| [[Triple point]] | |||
| 237.2 K (–35.9 °C), ? Pa | |||
|- | |||
| [[Critical point (chemistry)|Critical point]] | |||
| 561.6 K (288.5 °C), 5380 kPa | |||
|- | |||
| [[Standard enthalpy change of fusion|Std enthalpy change<br/>of fusion]], Δ<sub>fus</sub>''H''<sup><s>o</s></sup> | |||
| 8.8366 kJ/mol at –35.9 °C | |||
|- | |||
| [[Standard entropy change of fusion|Std entropy change<br/>of fusion]], Δ<sub>fus</sub>''S''<sup><s>o</s></sup> | |||
| 37.25 J/(mol·K) at –35.9 °C | |||
|- | |||
| [[Standard enthalpy change of vaporization|Std enthalpy change<br/>of vaporization]], Δ<sub>vap</sub>''H''<sup><s>o</s></sup> | |||
| 33.91 kJ/mol at 20°C | |||
|- | |||
| [[Standard entropy change of vaporization|Std entropy change<br/>of vaporization]], Δ<sub>vap</sub>''S''<sup><s>o</s></sup> | |||
| ? J/(mol·K) | |||
|- | |||
! {{chembox header}} | Solid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? J/(mol K) | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| ? J/(mol K) | |||
|- | |||
! {{chembox header}} | Liquid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>liquid</sub> | |||
| –169.7 kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>liquid</sub> | |||
| 208.53 J/(mol K) | |||
|- | |||
| [[Enthalpy of combustion]], Δ<sub>c</sub>''H''<sup><s>o</s></sup><sub>liquid</sub> | |||
| –1236.4 kJ/mol | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| 129.0 J/(mol K) | |||
|- | |||
! {{chembox header}} | Gas properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>gas</sub> | |||
| –125.4 kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>gas</sub> | |||
| ? J/(mol K) | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| 77.5 J/(mol K) at 25°C | |||
|- | |||
|} | |||
==Vapor pressure of liquid== | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| {{chembox header}} | '''P in mm Hg''' || 1 || 10 || 40 || 100 || 400 || 760 || 1520 || 3800 || 7600 || 15200 || 30400 || 45600 | |||
|- | |||
| {{chembox header}} | '''T in °C''' || –44.5<sub>(s)</sub> || –13.6 || 10.0 || 29.4 || 64.0 || 83.4 || 108.1 || 147.8 || 183.5 || 226.5 || 272.0 || — | |||
|} | |||
Table data obtained from ''CRC Handbook of Chemistry and Physics'' 44th ed. The (s) annotation indicates temperature is equilibrium of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. | |||
[[File:LogEthylenedichlorideVaporPressure.png|thumb|828px|left|'''log<sub>10</sub> of 1,2-dichloroethane vapor pressure.''' Uses formula: <math>\scriptstyle \log_e P_{mmHg} =</math><math>\scriptstyle \log_e (\frac {760} {101.325}) - 10.38248\log_e(T+273.15) - \frac {6904.904} {T+273.15} + 83.96795 + 8.368130 \times 10^{-6} (T+273.15)^2</math> obtained from CHERIC<ref name="cheric_p">{{Cite web|url=http://www.cheric.org/research/kdb/hcprop/cmpsrch.php|title=Pure Component Properties|format=Queriable database|publisher=Chemical Engineering Research Information Center|accessdate=5 June 2007| archiveurl= http://web.archive.org/web/20070603180431/http://www.cheric.org/research/kdb/hcprop/cmpsrch.php| archivedate= 3 June 2007 <!--Added by DASHBot-->}}</ref>]]{{Clear}} | |||
==Distillation data== | |||
See also: | |||
* [[tetrachloroethylene (data page)#Distillation data|Tetrachloroethylene (data page)]] | |||
{| | |||
|- valign="top" | |||
| | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| bgcolor="#D0D0D0" align=center colspan="3" | '''Vapor-liquid equilibrium<br>of 1,2-Dichloroethylene/<br>[[Methanol]]'''<ref name="cheric">{{Cite web|url=http://www.cheric.org/research/kdb/hcvle/hcvle.php|title=Binary Vapor-Liquid Equilibrium Data|publisher=Chemical Engineering Research Information Center|format=Queriable database|accessdate=5 Jun 2007| archiveurl= http://web.archive.org/web/20070829144547/http://www.cheric.org/research/kdb/hcvle/hcvle.php| archivedate= 29 August 2007 <!--Added by DASHBot-->}}</ref><br>''P'' = 96.7 kPa | |||
|- {{chembox header}} | |||
! rowspan="2" | BP<br>Temp.<br>°C | |||
! colspan="2" | % by mole methanol | |||
|- {{chembox header}} | |||
! liquid !! vapor | |||
|- | |||
| 63.3 || 100.00 || 100.00 | |||
|- | |||
| 60.2 || 92.01 || 82.50 | |||
|- | |||
| 59.3 || 79.52 || 72.06 | |||
|- | |||
| 58.9 || 69.03 || 66.43 | |||
|- | |||
| 59.1 || 63.50 || 63.50 | |||
|- | |||
| 59.0 || 59.02 || 63.27 | |||
|- | |||
| 59.3 || 47.91 || 63.25 | |||
|- | |||
| 59.6 || 43.02 || 62.55 | |||
|- | |||
| 61.8 || 15.03 || 54.15 | |||
|- | |||
| 66.7 || 6.01 || 42.03 | |||
|- | |||
| 82.15 || 0.00 || 0.0 | |||
|- | |||
|} | |||
| | |||
| | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| bgcolor="#D0D0D0" align=center colspan="3" | '''Vapor-liquid equilibrium<br>of 1,2-Dichloroethylene/<br>[[Ethanol]]'''<ref name="cheric"/><br>''P'' = 760 mm Hg | |||
|- {{chembox header}} | |||
! rowspan="2" | BP<br>Temp.<br>°C | |||
! colspan="2" | % by mole ethanol | |||
|- {{chembox header}} | |||
! liquid !! vapor | |||
|- | |||
| 79.9 || 3.0 || 13.0 | |||
|- | |||
| 78.0 || 4.2 || 19.5 | |||
|- | |||
| 76.7 || 7.3 || 23.3 | |||
|- | |||
| 74.1 || 11.3 || 31.5 | |||
|- | |||
| 72.6 || 15.2 || 36.0 | |||
|- | |||
| 70.9 || 28.3 || 43.6 | |||
|- | |||
| 70.5 || 34.5 || 45.2 | |||
|- | |||
| 70.2 || 42.5 || 47.8 | |||
|- | |||
| 70.1 || 48.8 || 49.8 | |||
|- | |||
| 70.2 || 59.8 || 53.5 | |||
|- | |||
| 70.8 || 70.5 || 58.2 | |||
|- | |||
| 71.4 || 76.5 || 62.8 | |||
|- | |||
| 72.1 || 81.7 || 67.0 | |||
|- | |||
| 74.7 || 92.3 || 81.7 | |||
|- | |||
| 75.7 || 94.8 || 86.5 | |||
|- | |||
| 77.3 || 97.8 || 94.5 | |||
|- | |||
|} | |||
| | |||
| | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| bgcolor="#D0D0D0" align=center colspan="3" | '''Vapor-liquid equilibrium<br>of 1,2-Dichloroethylene/<br>[[Toluene]]'''<ref name="cheric"/><br>''P'' = 760 mm Hg | |||
|- {{chembox header}} | |||
! rowspan="2" | BP<br>Temp.<br>°C | |||
! colspan="2" | % by mole dichloroethane | |||
|- {{chembox header}} | |||
! liquid !! vapor | |||
|- | |||
| 85.5 || 89.7 || 94.2 | |||
|- | |||
| 87.5 || 80.1 || 89.2 | |||
|- | |||
| 89.1 || 69.8 || 82.1 | |||
|- | |||
| 91.3 || 60.5 || 78.2 | |||
|- | |||
| 92.9 || 54.0 || 72.5 | |||
|- | |||
| 94.6 || 48.2 || 66.3 | |||
|- | |||
| 97.1 || 39.8 || 60.3 | |||
|- | |||
| 97.8 || 36.4 || 55.0 | |||
|- | |||
| 101.5 || 26.2 || 42.4 | |||
|- | |||
| 102.7 || 20.0 || 34.8 | |||
|- | |||
| 105.7 || 14.0 || 27.5 | |||
|- | |||
| 108.1 || 7.0 || 16.9 | |||
|- | |||
| 109.1 || 1.8 || 6.6 | |||
|- | |||
|} | |||
| | |||
| | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| bgcolor="#D0D0D0" align=center colspan="3" | '''Vapor-liquid equilibrium<br>of 1,2-Dichloroethylene/<br>[[Cyclohexane]]'''<ref name="cheric"/><br>''P'' = 760 mm Hg | |||
|- {{chembox header}} | |||
! rowspan="2" | BP<br>Temp.<br>°C | |||
! colspan="2" | % by mole cyclohexane | |||
|- {{chembox header}} | |||
! liquid !! vapor | |||
|- | |||
| 80.5 || 6.50 || 14.60 | |||
|- | |||
| 80.0 || 8.25 || 17.00 | |||
|- | |||
| 78.8 || 12.00 || 23.70 | |||
|- | |||
| 77.6 || 17.30 || 29.80 | |||
|- | |||
| 77.0 || 21.50 || 33.40 | |||
|- | |||
| 76.4 || 26.15 || 37.50 | |||
|- | |||
| 75.9 || 31.90 || 40.80 | |||
|- | |||
| 75.6 || 36.10 || 43.60 | |||
|- | |||
| 75.2 || 42.95 || 47.60 | |||
|- | |||
| 75.1 || 51.70 || 52.50 | |||
|- | |||
| 75.1 || 52.45 || 53.00 | |||
|- | |||
| 75.1 || 56.10 || 55.10 | |||
|- | |||
| 75.2 || 59.75 || 57.10 | |||
|- | |||
| 75.7 || 72.90 || 65.60 | |||
|- | |||
| 76.6 || 82.00 || 73.50 | |||
|- | |||
| 77.5 || 88.00 || 79.80 | |||
|- | |||
| 79.3 || 95.50 || 91.50 | |||
|- | |||
|} | |||
|} | |||
== Spectral data == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | [[UV/VIS spectroscopy|UV-Vis]] | |||
|- | |||
| [[Lambda-max|λ<sub>max</sub>]] | |||
| ? [[Nanometre|nm]] | |||
|- | |||
| [[molar absorptivity|Extinction coefficient]], ε | |||
| ? | |||
|- | |||
! {{chembox header}} | [[Infrared|IR]] | |||
|- | |||
| Major absorption bands | |||
| ? cm<sup>−1</sup> | |||
|- | |||
! {{chembox header}} | [[NMR Spectroscopy|NMR]] | |||
|- | |||
| [[Proton NMR]] <!-- Link to image of spectrum --> | |||
| | |||
|- | |||
| [[Carbon-13 NMR]] <!-- Link to image of spectrum --> | |||
| | |||
|- | |||
| Other NMR data <!-- Insert special data e.g. <sup>19</sup>F chem. shifts, omit if not used --> | |||
| | |||
|- | |||
! {{chembox header}} | [[Mass Spectrometry|MS]] | |||
|- | |||
| Masses of <br>main fragments | |||
| <!-- Give list of major fragments --> | |||
|- | |||
|} | |||
==References== | |||
<references/> | |||
* {{Cite web|url=http://webbook.nist.gov/chemistry/ | work = NIST Standard|title=Reference Database| accessdate= 4 June 2007 <!--Added by DASHBot-->| archiveurl= http://web.archive.org/web/20070531161144/http://webbook.nist.gov/chemistry/#Documentation| archivedate= 31 May 2007 <!--Added by DASHBot-->}} | |||
Except where noted otherwise, data relate to [[standard ambient temperature and pressure]]. | |||
[[wikipedia:Chemical infobox|Disclaimer]] applies. | |||
{{DEFAULTSORT:1,2-Dichloroethane (Data Page)}} | |||
[[Category:Chemical data pages|Dichloroethane]] | |||
Latest revision as of 06:56, 20 August 2013
30 year-old Entertainer or Range Artist Wesley from Drumheller, really loves vehicle, property developers properties for sale in singapore singapore and horse racing. Finds inspiration by traveling to Works of Antoni Gaudí. This page provides supplementary chemical data on 1,2-dichloroethane.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction,[1] nD | 1.4448 |
| Abbe number | ? |
| Dielectric constant,[2] εr | 10.5 ε0 at 20 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[3] | 40.05 mN/m at 10°C 38.75 mN/m at 20°C 28.4 mN/m at 100°C |
| Viscosity[4] | 1.1322 mPa·s at 0°C 0.8385 mPa·s at 20°C 0.6523 mPa·s at 40°C 0.4357 mPa·s at 80°C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 237.2 K (–35.9 °C), ? Pa |
| Critical point | 561.6 K (288.5 °C), 5380 kPa |
| Std enthalpy change of fusion, ΔfusH |
8.8366 kJ/mol at –35.9 °C |
| Std entropy change of fusion, ΔfusS |
37.25 J/(mol·K) at –35.9 °C |
| Std enthalpy change of vaporization, ΔvapH |
33.91 kJ/mol at 20°C |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–169.7 kJ/mol |
| Standard molar entropy, S |
208.53 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–1236.4 kJ/mol |
| Heat capacity, cp | 129.0 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–125.4 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 77.5 J/(mol K) at 25°C |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 |
| T in °C | –44.5(s) | –13.6 | 10.0 | 29.4 | 64.0 | 83.4 | 108.1 | 147.8 | 183.5 | 226.5 | 272.0 | — |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. The (s) annotation indicates temperature is equilibrium of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid.
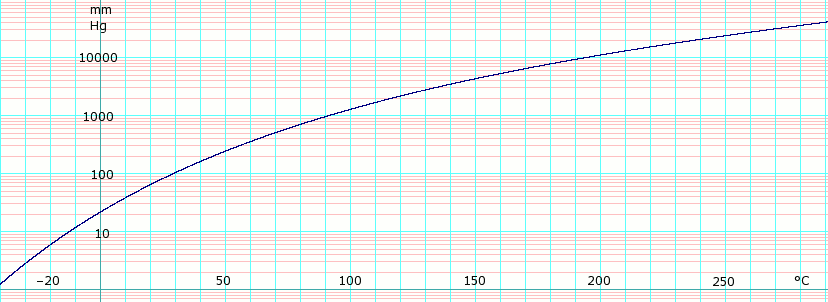
50 year old Petroleum Engineer Kull from Dawson Creek, spends time with interests such as house brewing, property developers in singapore condo launch and camping. Discovers the beauty in planing a trip to places around the entire world, recently only coming back from .
Distillation data
See also:
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
References
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Lange's Handbook of Chemistry, 10th ed. pp 1161-1163
- ↑ Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- ↑ Template:Cite web
- ↑ 6.0 6.1 6.2 6.3 Template:Cite web
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.